Pesticides >> Insecticides >> Rich-d-transallethrin
Rich-d-transallethrin 93%TC
NOMENCLATURE
Common name esdepallhrine (France (AFNOR))
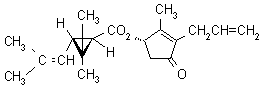
IUPAC name (S)-3-allyl-2-methyl-4-oxocyclopent-2-enyl (1R,3R)-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate
Alt: (S)-3-allyl-2-methyl-4-oxocyclopent-2-enyl (+)-trans-chrysanthemate
Roth: (S)-3-allyl-2-methyl-4-oxocyclopent-2-enyl (1R)-trans-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate
Chemical Abstracts name [1R-[1a(S*),3b]]-2-methyl-4-oxo-3-(2-propenyl)-2-cyclopenten-1-yl
2,2-dimethyl-3-(2-methyl-1-propenyl)cyclopropanecarboxylate
Other names S-bioallethrin; Esbiol CAS RN [28434-00-6] EEC no. 249-013-5
Development codes RU 3054; RU 16 121 (for OMS 3046); RU 27 436 (for
OMS 3045) (all Roussel Uclaf) Official codes AI3-29 024; OMS 3046
(for Esbiol); OMS 3045 (for Esbiothrin)
PHYSICAL CHEMISTRY
Composition Esbiol (OMS 3046) contains ?5% m/m total isomers, of
which ?0% is esdepallhrine.
Esbiothrin (OMS 3045) contains ?3% m/m total isomers, of which 2%
is esdepallhrine, the remainder being essentially the (R)-cyclopentenyl
isomer, with <3% cis- isomers. Mol. wt. 302.4 M.f. C19H26O3 Form
Orange-yellow viscous liquid. M.p. Not applicable; no crystallisation
observed at -40 B.p. 165-170 /0.15 mmHg (OMS 3046); 163-170 /0.15
mmHg (OMS 3045) V.p. 44 mPa (25 ) S.g./density 1.010 at 20 Solubility
Completely miscible with 100% ethanol, acetone, dichloromethane,
di-isopropyl ether, toluene, n-hexane, chloroform, ethyl acetate,
methanol, n-octanol, and petroleum solvent (20 ). Stability Degraded
by u.v. light. Specific rotation OMS 3046: [a]D20 -47.5?to -55?(50
g/l toluene); OMS 3045: [a]D20 ?37?(50 g/l toluene) F.p. 113 (open
cup)
COMMERCIALISATION
History Insecticide reported by F. Rauch et al. (Meded. Fac. Landbouwwet.
Rijksuniv. Gent, 1972, 37, 755). Introduced by Roussel Uclaf (now
Aventis). Manufacturers Aventis
APPLICATIONS
Mode of action Non-residual insecticide with rapid knockdown action.
Uses Esdepall hrine is by far the most potent constituent of allethrin.
OMS 3046 is used in aerosols, coils, electric vapourisers, or sprays
indoors against flying and crawling insects (Blattodea, Culicidae,
Muscidae). Also used in aerosols and sprays in mixtures with other
pyrethroids and piperonyl butoxide. OMS 3045 has similar uses to
OMS 3046. Formulation types AE; OL; UL; VP; Water-miscible liquid.
Selected tradenames: mixtures: 'Coopex' (+ permethrin) (Kwizda)
OTHER TRADENAMES
'Resigen' (Aventis); 'Reslin' (Aventis) mixtures: 'Aqua Reslin'
(+ permethrin) (Aventis); 'Crackdown' (+ deltamethrin) (environmental
health uses) (Aventis); 'Detrans' (+ bioallethrin+ bioresmethrin)
(Aventis); 'Dorine' (+ bioallethrin+ bioresmethrin) (Aventis); 'K-O'
(+ bioallethrin+ bioresmethrin) (Aventis); 'Neopybuthrin' (+ bioallethrin+
permethrin) (Aventis)
ANALYSIS
Product analysis of pyrethroids reviewed by E. Papadopoulou-Mourkidou
in Comp. Analyt. Profiles. By glc (CIPAC Handbook, 1980, 1A, 1097);
proportions of stereoisomers determined by glc of suitable derivatives
(A. Murano, Agric. Biol. Chem., 1972, 36, 2203; A. Horiba et al.,
ibid., 1977, 41, 2003; 1978, 42, 671; F. E. Rickett, Analyst (London),
1973, 98, 687) or by nmr (F. E. Rickett & P. B. Henry, ibid.,
1974, 99, 330). Residues determined by glc (D. B. McClellan, Anal.
Methods Pestic., Plant Growth Regul. Food Addit., 1964, 2, 25; Anal.
Methods Pestic. Plant Growth Regul., 1972, 6, 283; J. Sherma, ibid.,
1976, 8, 117).
MAMMALIAN TOXICOLOGY
Reviews A. J. Gray & D. M. Soderlund, Chapt. 5 in "Insecticides".
Oral Acute oral LD50 esdepall hrine:- for male rats 784, female
rats 1545 mg/kg. OMS 3045:- for male rats 432, female rats 378 mg/kg.
OMS 3046:- for male rats 574.5, female rats 412.9 mg/kg (in PEG
200). Skin and eye Acute percutaneous LD50 for rabbits:- 1545 mg
esdepall hrine/kg, >2000 mg OMS 3045/kg, >2000 mg OMS 3046/kg.
Inhalation LC50 (4 h) for rats c. 1.26 mg esdepall hrine/l air,
c. 2.63 mg OMS 3045/l air. NOEL Esdepall hrine:- (0.5 y) for rats
1000 mg/kg diet. OMS 3045:- (2 y) for rats 500 ppm; not an oncogen
up to 4500 mg/kg diet; (2 y) for mice 250 ppm; not an oncogen up
to 1250 mg/kg diet; (1 y) for dogs 400 ppm. Other No mutagenic,
carcinogenic, embryotoxic or teratogenic effects have been observed.
Toxicity class WHO (a.i.) II; EPA (formulation) III EC hazard Xn;
R20/22| N; R50, R53
ECOTOXICOLOGY
Birds Acute oral LD50 for mallard ducks and bobwhite quail >5000
mg/kg; for chickens >5000 mg esdepall hrine/kg. LC50 (8 d) for
ducks and quail >5000 mg/kg diet. Fish LC50 (96 h) for rainbow
trout 0.01, bluegill sunfish 0.033 mg/l. LC50 (96 h, static test)
for fathead minnow 0.0800, yellow perch 0.0078 mg/l.
ENVIRONMENTAL FATE
EHC 87 (WHO, 1989).
CAS NO. :28434-00-6