Products >> Herbicides >> Acetochlor
Acetochlor
Acetochlor 90%EC
Acetochlor 50%EC
Herbicide
HRAC K3 WSSA 15; chloroacetamide
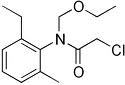
NOMENCLATURE
Common name acetochlor (BSI, E-ISO, ANSI, WSSA); acetochlore ((m)
F-ISO)
IUPAC name 2-chloro-N-ethoxymethyl-6'-ethylaceto-o-toluidide
Chemical Abstracts name 2-chloro-N-(ethoxymethyl)-N-(2-ethyl-6-methylphenyl)acetamide
CAS RN [34256-82-1] EEC no. 251-899-3 Development codes MON 097
(Monsanto)
Acetochlor APPLICATIONS
Biochemistry Inhibits cell division by blocking protein synthesis.
Maize tolerance of chloroacetamides is due mainly to conjugation
with glutathione; P450 metabolism may also be a factor. Mode of
action Selective herbicide, absorbed mainly by the shoots and secondarily
by the roots of germinating plants. Uses Used pre-emergence or pre-plant
to control annual grasses, certain annual broad-leaved weeds and
yellow nutsedge in maize (at 3 kg/ha), peanuts, soya beans, cotton,
potatoes and sugar cane. Formulation types CS; EC. Selected tradenames:
'Harness' (Monsanto); 'Acenit' ; mixtures: 'Sacemid A' (+ TI-35)
(North Hungarian); 'Surpass' (+ dichlormid) (Dow AgroSciences);
'Trophy' (+ dichlormid) (Dow AgroSciences)
Acetochlor ANALYSIS
Product by gc. Residues by hplc. Details from Monsanto.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats 2148 mg/kg. Skin and eye Acute percutaneous
LD50 for rabbits 4166 mg/kg. Contact sensitisation reactions observed
in guinea pigs. Practically non-irritating to eyes and skin (rabbits).
Inhalation LC50 (4 h) for rats >3.0 mg/l air. NOEL (2 y) for
rats 10 mg/kg b.w. daily; (1 y) for dogs 12 mg/kg b.w. daily. ADI
0.01 mg/kg b.w. Toxicity class WHO (a.i.) III EC hazard Xn; R20|
Xi; R37/38| R43| N; R50, R53
Acetochlor ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail 1260 mg/kg. LC50 (5 d)
for quail and mallard ducks >5620 mg/kg. Fish LC50 (96 h) for
rainbow trout 0.36, bluegill sunfish 1.5 mg/l. Daphnia LC50 (48
h) 9 mg/l. Bees LD50 (24 h) (contact) >200 mg/bee; (oral) >100
mg/bee. Worms LC50 (14 d) 211 mg/kg.
ENVIRONMENTAL FATE
Animals The primary routes of metabolism are glutathione conjugation
and metabolism by cytochrome P450. Plants In maize and soya beans,
rapidly absorbed and metabolised in the germinating plant. In maize,
the first metabolite is glutathione, and in soya beans homoglutathione
(E. J. Breaux, J. Agric. Food Chem., 1986, 34, 884). Soil/Environment
Adsorbed by soil, with little leaching. Microbial degradation accounts
for most loss from soil; DT50 8-18 d.