Pesticides >> Insecticides >> Spinosad
Spinosad
Spinosad 95%TC
Spinosad 48%SC
Spinosad 5%SC
Spinosad 2.5%SC
Insecticide
IRAC 5; spinosyn
Spinosad NOMENCLATURE
Common name spinosad (BSI, pa ISO, ANSI)
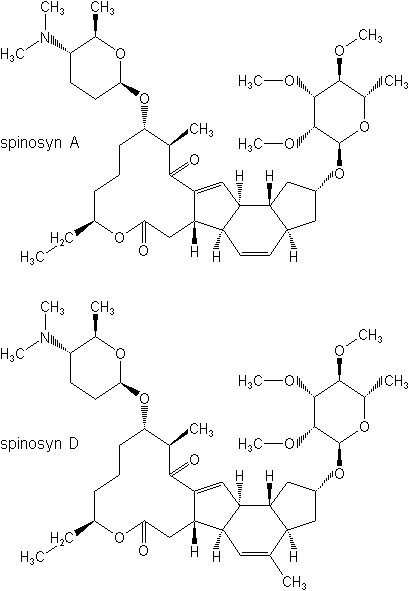
IUPAC name A mixture of (2R,3aR,5aR,5bS,9S,13S,14R,16aS,16bR)-2-(6-deoxy-2,3,4-tri-O-methyl-a-L-mannopyranosyloxy)-13-(4-dimethylamino-2,3,4,6-tetradeoxy-b-D-erythopyranosyloxy)-9-ethyl-2,3,3a,5a,5b,6,7,9,10,11,12,13,14,15,16a,16b-hexadecahydro-14-methyl-1H-8-oxacyclododeca[b]as-indacene-7,15-dione and (2R,3aS,5aR,5bS,9S,13S,14R,16aS,16bR)-2-(6-deoxy-2,3,4-tri-O-methyl-a-L-mannopyranosyloxy)-13-(4-dimethylamino-2,3,4,6-tetradeoxy-b-D-erythopyranosyloxy)-9-ethyl-2,3,3a,5a,5b,6,7,9,10,11,12,13,14,15,16a,16b-hexadecahydro-4,14-dimethyl-1H-8-oxacyclododeca[b]as-indacene-7,15-dione in the proportion 50-95% to 50-5%
Chemical Abstracts name 2-[(6-deoxy-2,3,4-tri-O-methyl-a-L-mannopyranosyl)oxy]-13-[[5-(dimethylamino)tetrahydro-6-methyl-2H-pyran-2-yl]oxy]-9-ethyl-2,3,3a,5a,5b,6,9,10,11,12,13,14,16a,16b-tetradecahydro-14-methyl-1H-as-indaceno(3,2-d)oxacyclododecin-7,15-dione (spinosyn A), mixture with 2-[(6-deoxy-2,3,4-tri-O-methyl-a-L-mannopyranosyl)oxy]-13-[[5-(dimethylamino)tetrahydro-6-methyl-2H-pyran-2-yl]oxy]-9-ethyl-2,3,3a,5a,5b,6,9,10,11,12,13,14,16a,16b-tetradecahydro-4,14-dimethyl-1H-as-indaceno(3,2-d)oxacyclododecin-7,15-dione (spinosyn D)
CAS RN [168316-95-8]; [131929-60-7] spinosyn A; [131929-63-0] spinosyn D Development codes XDE-105; DE-105 (both Dow)
Spinosad PHYSICAL CHEMISTRY
Composition Tech. is >90%, composed of 50-95% spinosyn A and 50-5% spinosyn D. Mol. wt. 732.0 (spinosyn A); 746.0 (spinosyn D) M.f. C41H65NO10 (spinosyn A); C42H67NO10 (spinosyn D) Form Light grey to white crystals (tech.). M.p. 84-99.5 °C (spinosyn A); 161.5-170 °C (spinosyn D) V.p. 3.0 ´ 10-5 mPa (25 °C) (spinosyn A); 2.0 ´ 10-5 mPa (25 °C) (spinosyn D) KOW logP = 2.8 (pH 5), 4.0 (pH 7), 5.2 (pH 9) (spinosyn A); logP = 3.2 (pH 5), 4.5 (pH 7), 5.2 (pH 9) (spinosyn D) S.g./density 0.512 (bulk, 20 °C) Solubility Spinosyn A: In water 89 ppm (distilled water), 235 ppm (pH 7) (both 20 °C). In acetone 16.8, acetonitrile 13.4, dichloromethane 52.5, hexane 0.448, methanol 19.0, n-octanol 0.926, toluene 45.7 (all in g/l, 20 °C). Spinosyn D: In water 0.5 ppm (distilled water), 0.33 ppm (pH 7) (both 20 °C). In acetone 1.01, acetonitrile 0.255, dichloromethane 44.8, hexane 0.743, methanol 0.252, n-octanol 0.127, toluene 15.2 (all in g/l, 20 °C). Stability Stable to hydrolysis at pH 5 and 7; DT50 (pH 9) 200 d (spinosyn A), 259 d (spinosyn D). Aquatic photodegradation DT50 (pH 7) 0.93 d (spinosyn A), 0.82 d (spinosyn D). pKa 8.1 (spinosyn A); 7.87 (spinosyn D)
Spinosad COMMERCIALISATION
Production Derived from the actinomycete Saccharopolyspora spinosa. Following fermentation, spinosad is obtained from a whole broth extraction. History The spinosyns first identified, in a soil sample, by Eli Lilly & Co. (agrochemical interests now Dow AgroSciences) in 1982. Commercially introduced during 1997. Patents US 5202242; EP 375316 Manufacturers Dow AgroSciences
Spinosad APPLICATIONS
Biochemistry Activation of the nicotinic acetylcholine receptor, but at a different site from nicotine or imidacloprid. Mode of action Active by contact and ingestion; causes paralysis. Uses For control of pest Lepidoptera (e.g. Ostrinia nubilalis, Helicoverpa zea, Trichoplusia ni, Plutella xylostella, Spodoptera spp., Heliothis spp., Pieris rapae, Keiferia lycopersicella, Lobesia botrana), thrips (e.g. Frankliniella occidentalis, Thrips palmi), flies (e.g. Liriomyza spp., Ceratitis capitata), beetles (e.g. Leptinotarsa decemlineata) and grasshoppers in cotton, row crops, vegetables, and fruits, at 4.8-36 g/hl. Also used for urban pest control (e.g. Agrotis ipsilon, Spodoptera spp., Parapediasia teterella) in turf and ornamentals, for structural control of drywood termites (e.g. Cryptotermes brevis, Incisitermes snyderi), and for fire ant (Solenopsis spp.) control. Effective as a bait for fruit flies (Ceratitis spp., Bactrocera spp., etc) and some ants (Solenopsis spp.).Under development for use on livestock animals for control of chewing and sucking lice (e.g. Linognathus vituli, Bovicola ovis, Solenopotes capillatus) and flies (e.g. Haematobia irritans, Lucilia cuprina), and in livestock premises for control of nuisance flies (e.g. Stomoxys calcitrans, Musca domestica, H. irritans). Formulation types SC; WG. Selected products: 'Conserve' (Dow AgroSciences); 'SpinTor' (Dow AgroSciences); 'Success' (Dow AgroSciences); 'Tracer' (Dow AgroSciences)