Products >> Herbicides >> Metsulfuron-methyl
Metsulfuron-methyl
Metsulfuron-methyl 95%TC
Metsulfuron-methyl 60%WG
Metsulfuron-methyl 20%WG
Herbicide
HRAC B WSSA 2; sulfonylurea
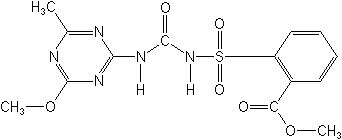
Metsulfuron-methyl NOMENCLATURE
Chemical Abstracts name methyl 2-[[[[(4-methoxy-6-methyl-1,3,5-triazin-2-yl)amino]carbonyl]amino]sulfonyl]benzoate
CAS RN [74223-64-6] Development codes DPX-T6376 (Du Pont)
metsulfuron
Common name metsulfuron (BSI, WSSA, ANSI, draft E-ISO, (m) draft
F-ISO)
IUPAC name 2-(4-methoxy-6-methyl-1,3,5-triazin-2-ylcarbamoylsulfamoyl)benzoic
acid
Chemical Abstracts name 2-[[[[(4-methoxy-6-methyl-1,3,5-triazin-2-yl)amino]carbonyl]amino]sulfonyl]benzoic
acid
CAS RN [79510-48-8]
Metsulfuron-methyl APPLICATIONS
metsulfuron-methyl
Biochemistry Branched chain amino acid synthesis (ALS or AHAS) inhibitor.
Acts by inhibiting biosynthesis of the essential amino acids valine
and isoleucine, hence stopping cell division and plant growth. Selectivity
derives from rapid metabolism in the crop. Metabolic basis of selectivity
in sulfonylureas reviewed (M. K. Koeppe & H. M. Brown, Agro-Food-Industry,
6, 9-14 (1995)). Mode of action Selective systemic herbicide absorbed
through the roots and foliage, with rapid translocation both acropetally
and basipetally. Susceptible plants cease growth almost immediately
after post-emergence treatment, and are killed in 7-21 days. Surfactants
increase the activity of metsulfuron-methyl on certain broad-leaved
weeds. Uses Metsulfuron-methyl controls a wide range of annual and
perennial broad-leaved weeds in wheat, barley, rice and oats, by
either pre- or post-emergence application, at 4-7.5 g/ha post-emergence.
Formulation types WG. Selected tradenames: 'Ally' (Du Pont); 'Malban'
(IPESA); 'Quit' (Sanonda); 'Retador' (Reposo)
Metsulfuron-methyl OTHER TRADENAMES
'Alli? (Du Pont); 'Escort' (Du Pont); 'Gropper' (Du Pont); 'Jubilee'
(Du Pont); 'Lorate' (Du Pont); 'Luger' (Chemiplant); 'Pilarcort'
(Pilarquim); 'Triticas' (CAS) mixtures: 'Alli?Express' (+ carfentrazone-ethyl)
(Du Pont); 'Ally Express' (+ carfentrazone-ethyl) (Du Pont); 'Canvas'
(+ THIFENSULFURON-METHYL+ TRIBENURON-METHYL) (Du Pont); 'Finesse'
(+ chlorsulfuron) (Du Pont); 'Harmony M' (+ thifensulfuron-methyl)
(Du Pont); 'Lexus XPE' (+ flupyrsulfuron-methyl-sodium) (Du Pont);
'Scoop' (+ thifensulfuron-methyl) (Du Pont); 'Sindax' (+ bensulfuron-methyl)
(Du Pont); 'Sulfonil' (+ propanil) (Crystal) Discontinued names:
'PartiSan' * (Sanonda)
Metsulfuron-methyl ANALYSIS
Product analysis by hplc (L. W. Hershberger & D. E. Brennan,
Anal. Methods Pestic. Plant Growth Regul., 1988, 16, 83). Residues
determined by hplc (idem, ibid.). Methods for sulfonylurea residues
in crops, soil and water reviewed (A. C. Barefoot et al., Proc.
Br. Crop Prot. Conf. - Weeds, 1995, 2, 707).
Metsulfuron-methyl MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for male and female rats >5000 mg/kg. Skin
and eye Acute percutaneous LD50 for rabbits >2000 mg/kg. Mild
skin irritant to guinea pigs, but not a skin sensitiser; moderate
but reversible eye irritant. Inhalation LC50 (4 h) for male and
female rats >5 mg/l air. NOEL (2 y) for rats 500, male dogs 500,
female dogs 5000 mg/kg diet. ADI 0.25 mg/kg (Germany). Other Non-mutagenic
in the Ames test. Non-teratogenic. Toxicity class WHO (a.i.) III
(Table 5); EPA (formulation) IV
metsulfuron
EC hazard N; R50, R53
ECOTOXICOLOGY
metsulfuron-methyl
Birds Acute oral LD50 for mallard ducks >5000 mg/kg. Dietary
LC50 (8 d) for mallard ducks and bobwhite quail >5620 mg/kg diet.
Fish LC50 (96 h) for rainbow trout and bluegill sunfish >150
mg/l. Daphnia EC50 (48 h) >150 mg/l. Algae NOEC for green algae
100 mg/l. Other aquatic spp. NOEC for Lemna gibba 0.16 mg/l. Bees
Non-toxic to bees; LD50 >25 mg/bee. Worms >1000 mg/kg.
ENVIRONMENTAL FATE
metsulfuron-methyl
Animals In mammals, following oral administration, metsulfuron-methyl
is excreted predominantly unchanged. The methoxycarbonyl and sulfonylurea
groups are only partly degraded, by O-demethylation and hydroxylation.
Plants In plants, undergoes complete degradation within a few days,
by hydrolysis and conjugation. In addition to the hydroxymethyl
analogue, other metabolites identified include methyl 2-(aminosulfonyl)benzoate
and 2-(aminosulfonyl)benzoic acid. Rapidly metabolised within cereal
plants. Soil/Environment In soil, metsulfuron-methyl is broken down
both by chemical hydrolysis and by microbial degradation. DT50 varies
from 1 to 5 weeks, with breakdown being more rapid at lower soil
pH, at higher temperatures, and at higher levels of soil moisture.
Koc 35 (pH 7).