Products >> Fungicides >> Kresoxim-methyl
Kresoxim-methyl
Kresoxim-methyl 95%TC
Kresoxim-methyl 50%WG
Kresoxim-methyl 50%SC
Fungicide
FRAC 11; strobilurin type: oximinoacetate
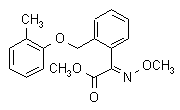
NOMENCLATURE
Common namekresoxim-methyl (BSI, pa ISO)
IUPAC namemethyl (E)-methoxyimino[2-(o-tolyloxymethyl)phenyl]acetate
Chemical Abstracts namemethyl (aE)-a-(methoxyimino)-2-[(2-methylphenoxy)methyl] benzeneacetate
CAS RN[143390-89-0]Development codesBAS 490F (BASF)
Kresoxim-methyl PHYSICAL CHEMISTRY
Mol. wt.313.4M.f.C18H19NO4FormWhite, mildly aromatic crystals.M.p.98-100 °CV.p.2.3 ´ 10-3mPa (20 ºC)KOWlogP = 3.4 (pH 7, 25 ºC)Henry3.6 ´ 10-4Pa m3mol-1S.g./density1.258 kg/l (20 °C)SolubilityIn water 2 mg/l (20 ºC).StabilityHydrolysis DT5034 d (pH 7), 7 h (pH 9); relatively stable at pH 5.pKaNone in range 2-12
Kresoxim-methyl COMMERCIALISATION
HistoryDiscovered by BASF AG in 1983. Reported by E. Ammermann et al. (Proc. Br. Crop Prot. Conf. - Pests Dis., 1992, 1, 403). In production since 1995 and introduced in 1996.PatentsEP 253213; US 4829085ManufacturersBASF
Kresoxim-methyl APPLICATIONS
BiochemistryInhibits mitochondrial respiration by blocking electron transfer between cytochrome b and cytochrome c1, at the ubiquinol oxidising site. Selectivity is partly due to enzymic de-esterification within plants.Mode of actionFungicide with protective, curative, eradicative and long residual disease control; acts by inhibiting spore germination. Redistribution via the vapour phase contributes to activity.UsesControl of scab in apples and pears (Venturia spp.); powdery mildew on apples (Podosphaera leucotricha), vines (Uncinula necator), cucurbits (Sphaerotheca fuliginea) and sugar beet (Erysiphe betae); mildew (Erysiphe graminis), scald (Rhynchosporium secalis), net blotch (Pyrenophora teres) and glume blotch (Septoria nodorum) on cereals; mildew on vegetables (Leveillula taurica, Erysiphe spp., Alternaria spp.).Formulation typesSC; SE; WG.Selected tradenames:'Stroby' (BASF, Nissan); mixtures:'Allegro' (+ epoxiconazole) (BASF); 'Mentor' (+ fenpropimorph) (BASF)
Kresoxim-methyl MAMMALIAN TOXICOLOGY
ReviewsFAO/WHO 83, 85 (see part 2 of the Bibliography).OralAcute oral LD50for rats >5000 mg/kg.Skin and eyeAcute percutaneous LD50for rats >2000 mg/kg. Non-irritating to skin and eyes (rabbits).InhalationLC50(4 h) for rats >5.6 mg/l.NOEL(3 mo) for male rats 2000 ppm (146 mg/kg daily), female rats 500 ppm (43 mg/kg daily).ADI(JMPR) 0.4 mg/kg b.w. [1998].OtherNegative in the Ames test, and non-teratogenic.
Kresoxim-methyl ECOTOXICOLOGY
Although it is toxic to aquatic species, exposure tests and ecological studies have shown that there is no danger of permanent damage to aquatic organisms when kresoxim-methyl is used as recommended.BirdsLD50(14 d) for quail >2150 mg/kg. LC50(8 d) for bobwhite quail and mallard ducks >5000 ppm.FishLC50(96 h) for bluegill sunfish 0.499 mg/l, rainbow trout 190 ppb.DaphniaEC50(48 h) 0.186 mg/l.AlgaeEC50(0-72 h) 63 mg/l.BeesLD50(48 h) (contact) >20 mg/bee.WormsLC50>937 mg/kg.