Pesticides >> Insecticides >> Etofenprox
Etofenprox
Etofenprox 95%TC,
Etofenprox 20%EC
Etofenprox 20%WP
Etofenprox 10%EC
Etofenprox 10%WP
Insecticide
non-ester pyrethroid
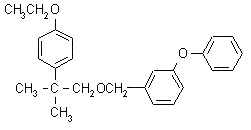
Etofenprox NOMENCLATURE
Common name etofenprox ((m) draft F-ISO, INN; BSI, draft E-ISO both
since 1988); ethofenprox* (before 1988)
IUPAC name 2-(4-ethoxyphenyl)-2-methylpropyl 3-phenoxybenzyl ether
Chemical Abstracts name 1-[[2-(4-ethoxyphenyl)-2-methylpropoxy]methyl]-3-phenoxybenzene
CAS RN [80844-07-1] Development codes MTI-500 (Mitsui) Official
codes OMS 3002
Etofenprox APPLICATIONS
Mode of action Insecticide with contact and stomach action. Uses
Control of rice water weevils, skippers, leaf beetles, leafhoppers,
planthoppers, and bugs on paddy rice; and aphids, moths, butterflies,
whitefly, leaf miners, leaf rollers, leafhoppers, thrips, borers,
etc. on pome fruit, stone fruit, citrus fruit, tea, soya beans,
sugar beet, brassicas, cucumbers, aubergines, and other crops. Also
used to control public health pests, and on livestock. Formulation
types CS; DP; EW; EC; GR; SL; UL; WP. Selected tradenames: 'Trebon'
(Mitsui)
Etofenprox OTHER TRADENAMES
'Vectron' (Mitsui); 'Nukil' (Mitsui, Dhanuka); 'Primo' (RPG) mixtures:
'Kasu-rab-validatrebon' (+ kasugamycin hydrochloride hydrate+ phthalide+
validamycin) (Hokko); 'Mon-Rab Trebon F' (+ flutolanil+ phthalide)
(Nihon Nohyaku); 'Vicidi-M' (+ phenthoate) (Vipesco)
ANALYSIS
Product analysis by glc with FID (CIPAC Handbook, 1995, G, 56-61).
Residues determined by glc with ECD.
Etofenprox MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 68, 70 (see part 2 of the Bibliography). Oral Acute
oral LD50 for male and female rats >42 880, male mice >107
200, dogs >5000 mg/kg. Skin and eye Acute percutaneous LD50 for
rats and male and female mice >2140 mg/kg; non-irritating to
skin and eyes (rabbits). Inhalation LC50 (4 h) for rats 5900 mg/m3.
NOEL (1 y) for dogs 32 mg/kg diet; (2 y) for male rats 3.7, female
rats 4.8, male mice 3.1, female mice 3.6 mg/kg diet. In life-span
feeding studies on rats and mice, at doses up to 4900 ppm, and on
dogs, at doses up to 10 000 ppm, no adverse effects were observed.
Mutagenicity, teratogenicity, and three-generation reproduction
studies showed no noticeable abnormalities. ADI (JMPR) 0.03 mg/kg
b.w. [1993]. Toxicity class WHO (a.i.) III (Table 5); EPA (formulation)
IV
Etofenprox ECOTOXICOLOGY
Birds Acute oral LD50 for mallard ducks >2000 mg/kg. Fish TLm
(48 h) for carp 5.0 ppm. Daphnia LC50 (3 h) >40 mg/l. Worms LC50
for earthworms (7 d) 43.1 ppm, (14 d) 24.6 ppm.
Etofenprox ENVIRONMENTAL FATE
Animals In rats, parent compound, 2-(4-hydroxyphenyl)-2-methylpropyl
3-(phenoxybenzyl) ether, and 2-(4-ethoxyphenyl)-2-methylpropyl 3-(4-hydroxybenzyl)
ether are found. Plants In rice, the main metabolite is 2-(4-ethoxyphenyl)-2-methylpropyl
3-phenoxybenzoate. Soil/Environment DT50 in soil c. 6 d.