Pesticides >> Insecticides >> Dimethoate
Dimethoate
Dimethoate 95%TC
Dimethoate 5%WG
Dimethoate 1%EC
Insecticide, acaricide
IRAC 1B; organophosphate
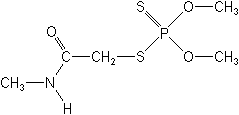
Dimethoate NOMENCLATURE
Common name dimethoate (BSI, E-ISO, (m) F-ISO, ANSI, ESA, JMAF); fosfamid (USSR)
IUPAC name O,O-dimethyl S-methylcarbamoylmethyl phosphorodithioate; 2-dimethoxyphosphinothioylthio-N-methylacetamide
Chemical Abstracts name O,O-dimethyl S-[2-(methylamino)-2-oxoethyl] phosphorodithioate
CAS RN [60-51-5] EEC no. 200-480-3 Development codes EI 12 880 (Cyanamid); L 395 (Montedison); BAS 152J (BASF); CME 103 (Celamerck) Official codes OMS 94; OMS 111; ENT 24 650
Dimethoate PHYSICAL CHEMISTRY
Composition Tech. grade is 95% pure. Mol. wt. 229.3 M.f. C5H12NO3PS2 Form Colourless crystals; (tech., white solid pellets). M.p. 50.0-51.5 °C (purity 99.5%) B.p. 117 ºC/0.1 mmHg V.p. 0.25 mPa (25 °C) KOW logP = 0.704 Henry 1.42 10-6 Pa m3 mol-1 S.g./density 1.31 (20 °C, 99.1% pure) Solubility In water 23.3 (pH 5), 23.3 (pH 7), 25.0 (pH 9) (all in g/l, 25 ºC). Readily soluble in most organic solvents, e.g. in alcohols, ketones, benzene, toluene, chloroform, dichloromethane >300, carbon tetrachloride, saturated hydrocarbons, n-octanol >50 (all in g/kg, 20 ºC). Stability Relatively stable in aqueous media at pH 2-7. Hydrolysed in alkaline solutions; DT50 4.4 d (pH 9). Photostability DT50 >175 d (pH 5). Decomposes on heating, forming the O,S-dimethyl analogue. pKa 2.0 (20 °C) Other properties Surface tension 47.8 mN/m.
Dimethoate COMMERCIALISATION
History Insecticide reported by E. I. Hoegberg & J. T. Cassaday (J. Am. Chem. Soc., 1951, 73, 557; Ital. Agric., 1955, 92, 747). Introduced by American Cyanamid Co. (who sold rights to Wilbur Ellis), by BASF AG, by Boehringer Sohn (now BASF AG), and by Montecatini S.p.A. (now Isagro S.p.A). Patents US 2494283 to Cyanamid; DE 1076662 to Celamerck; GB 791824 to Montedison Manufacturers Agrochem; Cheminova; Drexel; Mico; Rallis; Sannong; Shaw Wallace; Sundat
Dimethoate APPLICATIONS
Biochemistry Cholinesterase inhibitor. Mode of action Systemic insecticide and acaricide with contact and stomach action. Uses Control of a wide range of Acari, Aphididae, Aleyrodidae, Coccidae, Coleoptera, Collembola, Diptera, Lepidoptera, Pseudococcidae and Thysanoptera in cereals, citrus, coffee, cotton, fruit, grapes, olives, pastures, beetroot, potatoes, pulses, tea, tobacco, ornamentals, ornamental shrubs, and vegetables. Also used for control of flies in animal houses. Typical application rates for cereals 340-680, citrus 2100, olives 720, beetroot 84-600, vegetables 330 -600 (all in g/ha per application). Phytotoxicity Non-phytotoxic when used as directed, except to some varieties of lemon, peach, fig, olive, walnut, hop, tomato, bean, cotton, and pine. Russetting is possible with Red Delicious and Golden Delicious apples, and with some ornamentals. Phytotoxicity is dependent on crop variety and climate. Formulation types EC; GR; UL; WP; Aerosol.