Pesticides >> Insecticides >> Deltamethrin
Deltamethrin
Deltamethrin 98%TC
Deltamethrin 5%EC
Deltamethrin 2.5%EC
Insecticide
pyrethroid
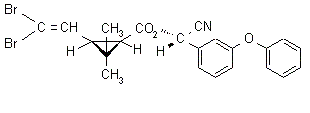
NOMENCLATURE
Common name deltamethrin (BSI, draft E-ISO); deltamethrine ((f)
draft F-ISO)
IUPAC name (S)-a-cyano-3-phenoxybenzyl (1R,3R)-3-(2,2-dibromovinyl)-2,2-dimethylcyclopropanecarboxylate
Roth: (S)-a-cyano-3-phenoxybenzyl (1R)-cis-3-(2,2-dibromovinyl)-2,2-dimethylcyclopropanecarboxylate
Chemical Abstracts name [1R-[1a(S*),3a]]-cyano(3-phenoxyphenyl)methyl
3-(2,2-dibromoethenyl)-2,2-dimethylcyclopropanecarboxylate
Other names decamethrin* (rejected common name proposal) CAS RN
[52918-63-5]; [52820-00-5] ((RS)- (1R)-cis- isomer pair) EEC no.
258-256-6 Development codes NRDC 161; RU 22 974 (Roussel Uclaf);
AE F032640 (AgrEvo) Official codes OMS 1998
Deltamethrin APPLICATIONS
Biochemistry Like all pyrethroids, prevents the sodium channels
from functioning, so that no transmission of nerve impulses can
take place. Mode of action Non-systemic insecticide with contact
and stomach action. Fast-acting. Uses A potent insecticide, effective
by contact and ingestion against a wide range of pests. Crop protection
uses include: Coleoptera (2.5-7.5 g/ha), Heteroptera (5.0-7.5 g/ha),
Homoptera (6.2-12.5 g/ha), Lepidoptera (5.0-21 g/ha) and Thysanoptera
(5-10 g/ha) in cereals, citrus, cotton, grapes, maize, oilseed rape,
soya beans, top fruit and vegetables. It controls Acrididae (5.0-12.5
g/ha), and is recommended against locusts. Soil surface sprays (2.5-5.0
g/ha) control Noctuidae. It is used against indoor crawling and
flying insects (12.5 mg/m2) and pests of stored grain (0.25-0.5
g/t) and timber (Blattodea, Culicidae, Muscidae). Dip or spray (12.5-75
mg/l), and pour-on (0.75 mg/kg b.w.) applications give good control
of Muscidae, Tabanidae, Ixodidae and other Acari on cattle, sheep
and pigs, etc. Formulation types DP; EC; EG; EW; GR; HN; PO; SC;
SL; TB; UL; WG; WP. Selected tradenames: 'Decis' (crop protection
uses) (Aventis); 'Kordon' (environmental health uses) (Aventis);
'K-Othrine' (environmental health uses) (Aventis); 'Butox' (veterinary
uses) (Intervet); 'Delta' (Nagarjuna Agrichem); 'Deltamix' (Agrimix);
'Sadethrin' (Sanonda)
Deltamethrin OTHER TRADENAMES
'Bitam' (crop protection uses) (Aventis); 'Cislin' (environmental
health uses) (Aventis); 'Decistab' (Aventis); 'Delsekte' (environmental
health uses) (Aventis); 'K-Otek' (environmental health uses) (Aventis);
'Pearl Micro' (Aventis); 'Tess' (crop protection uses) (Aventis);
'Delete' (Vapco); 'Deltarin' (Vapco); 'Deltarocca' (Rocca); 'Flayethrin'
(Agrochem); 'Keshet' (Makhteshim-Agan); 'Terminator' (Vapco); 'Thripstick'
(Aquaspersions); 'Thrust' (Crop Health) mixtures: 'Adage' (+ pirimicarb)
(Aventis); 'Akaridecis' (+ propargite) (Aventis); 'Best' (+ pirimicarb)
(Aventis); 'Crackdown' (+ bioallethrin S-cyclopentenyl isomer) (environmental
health uses) (Aventis); 'Dadeci' (+ buprofezin) (Aventis); 'Decis
D' (+ dimethoate) (Aventis); 'Decisdan' (+ endosulfan) (Aventis);
'Decisprime' (+ chlorpyrifos-methyl) (Aventis); 'Decisquick' (+
heptenophos) (Aventis); 'Deltaphos' (+ triazophos) (Aventis); 'Evidence'
(+ pirimicarb) (Aventis); 'K-obiol' (+ piperonyl butoxide) (Aventis);
'Patriot' (+ pirimicarb) (Aventis); 'Mieling' (+ phoxim) (Zhong-Xi)
Discontinued names: 'Landgold Deltaland' * (Landgold); 'Sputop'
* (Coopers)
ANALYSIS
Product analysis by lc with u.v. detection (AOAC Methods, 1995,
991.03, 7.5.05) or by hplc (CIPAC Handbook, 1988, D, 57; M. Vaysse
et al., Anal. Methods Pestic. Plant Growth Regul., 1984, 13, 53).
Residues determined by glc with ECD (Man. Pestic. Residue Anal.,
1987, I, 6, S19; Anal. Methods Residues Pestic., 1988, Part I, M11;
A. Ambrus et al., J. Assoc. Off. Anal. Chem., 1981, 64, 733; P.
G. Baker & P. Bottomley, Analyst (London), 1982, 107, 206; P.
Bottomley & P. G. Baker, ibid., 1984, 109, 85).
MAMMALIAN TOXICOLOGY
Reviews See A. J. Gray & D. M. Soderlund, Chapt. 5 in "Insecticides".
FAO/WHO 89, 91 (see part 2 of the Bibliography). IARC ref. 53 class
3 Oral Acute oral LD50 for rats ranges from 135->5000 mg/kg,
depending upon carrier and conditions of the study; for dogs >300
mg/kg. Acute oral LD50 for formulations in rats: >2000 mg (of
15 g/l EC)/kg; 445 mg (of 25 g/l EC)/kg; >5000 mg (of 5 g/l UL)/kg;
>16 000 mg (of 25 g/kg WP)/kg; >40 000 mg (of 25 g/l SC)/kg.
Skin and eye Acute percutaneous LD50 for rats and rabbits >2000
mg/kg. Non-irritating to skin; mild eye irritant (rabbits). Inhalation
LC50 (4 h) for rats 2.2 mg/l air; (1 h) >4.6 mg/l air (micronised).
NOEL (2 y) for mice 12, rats 1, dogs 1 mg/kg b.w. ADI (JMPR) 0.01
mg/kg b.w. [2000]. Other Non-mutagenic and non-teratogenic (mice,
rats, rabbits). Toxicity class WHO (a.i.) II; EPA (formulation)
II
ECOTOXICOLOGY
Birds Acute oral LD50 for mallard ducks >4640 mg/kg. Dietary
LC50 (8 d) for mallard ducks >8039, quail >5620 mg/kg diet.
NOEL for reproduction for mallard ducks >70, bobwhite quail >55
mg/kg daily. Fish Toxic to fish under laboratory conditions; LC50
(96 h) for rainbow trout 0.91, bluegill sunfish 1.4 mg/l. Not toxic
to fish under natural conditions. Daphnia LC50 (48 h) 3.5 mg/l.
Algae EC50 (96 h) for algae (Selenastrum capricornutum) >9.1
mg/l. Low LD50 and LC50 values under laboratory conditions do not
represent a significant hazard to aquatic fauna in normal field
use. Bees Toxic to bees, LD50 (oral) 79 ng/bee; (contact) 51 ng/bee.
Low LD50 and LC50 values under laboratory conditions do not represent
a significant hazard to bees in normal field use. Worms LC50 (14
d) for earthworms >1290 mg/kg soil.
ENVIRONMENTAL FATE
EHC 97 (WHO, 1990). Animals In rats, following oral administration,
elimination occurs within 2-4 days. The phenyl ring is hydroxylated,
the ester bond hydrolysed, and the acid moiety is eliminated as
the glucuronide and glycine conjugates. Plants No uptake through
leaves and roots - non-systemic compound. No major metabolites,
except in oily crops, where trans-deltamethrin is part of the residue
definition. Soil/Environment In soil, undergoes microbial degradation
within 1-2 weeks. Kd 3790-30 000, Koc 4.6 ´ 105-1.63 ´
107 cm3/g, confirms strong adsorption by soil colloids and no risk
of leaching. DT50 (lab., aerobic) 21-25 d, (anaerobic) 31-36 d.
In field, DT50 <23 d. Soil photolysis DT50 9 d. No incidence
on soil microflora and nitrogen cycle.