Pesticides >> Insecticides >> Clofentezine
Clofentezine 50%SC
Acaricide
tetrazine
NOMENCLATURE
Common name clofentezine (BSI, ANSI, draft E-ISO, (f) draft F-ISO)
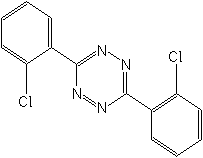
IUPAC name 3,6-bis(2-chlorophenyl)-1,2,4,5-tetrazine
Chemical Abstracts name 3,6-bis(2-chlorophenyl)-1,2,4,5-tetrazine
Other names bisclofentezin* (rejected common name proposal) CAS
RN [74115-24-5] EEC no. 277-728-2 Development codes NC 21 314 (Fisons)
Clofentezine APPLICATIONS
Mode of action Specific acaricide with contact action, and long
residual activity. Inhibits embryo development. Uses Control of
eggs and young motile stages (but not adults) of Panonychus ulmi
and Tetranychus spp. on pome fruit and stone fruit (20 g/hl), citrus
fruit (12.5-20 g/hl), nuts, vines (20 g/hl), hops, strawberries
(200-300 g/ha), cucurbits (100-400 g/ha), cotton (150-250 g/ha),
and ornamentals (20-30 g/hl). Has no effect on predatory mites or
beneficial insect species. Phytotoxicity May cause slight injury
to glasshouse roses. May cause a slight pink deposit on petals of
white or pale flowers. Formulation types SC; WP. Selected tradenames:
'Apollo' (Aventis)
Clofentezine OTHER TRADENAMES
'Acaristop' (Aventis); 'Apolo' (Aventis); 'Cara' (Aventis); 'Niagara'
(Rocca)
ANALYSIS
Product analysis by rp hplc (CIPAC Handbook, 1995, G, 18-23) or
by visible spectroscopy. Residues in plants and soil determined
by hplc. Details available from Aventis.
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 47, 49 (see part 2 of the Bibliography). Oral Acute
oral LD50 for rats >5200 mg/kg. Skin and eye Acute percutaneous
LD50 for rats >2100 mg/kg. Mild eye and skin irritant. Inhalation
LC50 (4 h) for rats >9 mg/l air. NOEL (2 y) for rats 40 mg/kg
diet; (1 y) for dogs 50 mg/kg diet. ADI (JMPR) 0.02 mg/kg b.w. [1986].
Toxicity class WHO (a.i.) III (Table 5); EPA (formulation) III
ECOTOXICOLOGY
Birds Acute oral LD50 for mallard ducks >3000, bobwhite quail
>7500 mg/kg. Dietary LC50 (8 d) for mallard ducks and bobwhite
quail >2000 mg/kg diet. Fish LC50 (96 h) for rainbow trout >0.015,
bluegill sunfish >0.25 mg/l (limits of solubility). Daphnia LC50
(48 h) >1.45 mg/l (limit of solubility). Algae Not toxic to Scenedesmus
panonicus up to limit of solubility. Bees Acute LD50 (oral) >20
mg/bee; LC50 (contact) >1500 ppm.
ENVIRONMENTAL FATE
Animals In mammals, undergoes metabolism by hydroxylation and exchange
of the chlorine atoms on the rings for methylthio groups. Following
oral administration, excretion occurs within 24-48 hours in the
urine and faeces. Plants In metabolism studies, unchanged clofentezine
was the major extractable residue. Trace amounts (4%) of 2-chlorobenzonitrile,
the major photodegradation product, were also detected. Soil/Environment
In soil, the major degradation route leads to 2-chlorobenzoic acid,
and finally to CO2. DT50 in soil 65-85 d (15 ), 28-56 d (25 ), depending
upon soil type. However, in laboratory studies, no leaching occurs.
In water, 2-chlorobenzonitrile is the major product formed by hydrolysis
and photodegradation, with smaller amounts of other compounds. Low
solubility in water makes determination of soil adsorption constants
difficult.