Pesticides >> Insecticides >> Pyridaben
Pyridaben
Pyridaben 95%TC
Pyridaben 20%WP
Pyridaben 10%EC
Insecticide, acaricide
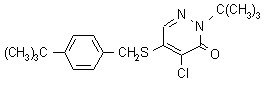
Pyridaben NOMENCLATURE
Common name pyridaben (BSI, draft E-ISO)
IUPAC name 2-tert-butyl-5-(4-tert-butylbenzylthio)-4-chloropyridazin-3(2H)-one
Chemical Abstracts name 4-chloro-2-(1,1-dimethylethyl)-5-[[[4-(1,1-dimethylethyl)phenyl]methyl]thio]-3(2H)-pyridazinone
CAS RN [96489-71-3] Development codes NC-129; NCI-129 (both Nissan);
BAS-300I (BASF)
Pyridaben APPLICATIONS
Biochemistry Inhibitor of mitochondrial electron transport at complex
I Mode of action Non-systemic insecticide and acaricide. Rapid knockdown
and long residual activity. Active against all developing stages,
especially against the larval and nymph stages. Uses Control of
Acari, Aleyrodidae, Aphididae, Cicadellidae and Thysanoptera on
field crops, fruit trees, ornamentals and vegetables, at 5-20 g/hl
or 100-300 kg/ha. Formulation types EC; SC; WP. Selected tradenames:
'Sanmite' (Nissan)
Pyridaben OTHER TRADENAMES
'Nexter' (Nissan); 'Dinomite' (Vapco); 'Pyramite' (Nissan, BASF);
'Samite' (Sanonda) Discontinued names: 'Poseidon' * (Nissan); 'Starling'
* (Nissan, Zeneca)
ANALYSIS
Details from Nissan.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for male rats 1350, female rats 820, male mice
424, female mice 383 mg/kg. Skin and eye Acute percutaneous LD50
for rats and rabbits >2000 mg/kg. Non-irritant to eyes and skin
(rabbits). Non-sensitising to skin (guinea pigs). Inhalation LC50
for male rats 0.66, female rats 0.62 mg/l air. NOEL (78 w) for mice
0.81 mg/kg daily; (52 w) for dogs 0.5 mg/kg daily. Other Non-mutagenic
in Ames, DNA repair, in vitro chromosomal (Chinese hamster), and
mouse micronucleus tests; non-teratogenic in rats and rabbits. Toxicity
class WHO (a.i.) III (20 WP); EPA (formulation) I (75 WP)
Pyridaben: ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail >2250, mallard ducks
>2500 mg/kg. Fish LC50 (96 h) for rainbow trout 1.1-3.1, bluegill
sunfish 1.8-3.3 mg/l; (48 h) for carp 8.3 mg/l. Daphnia EC50 (48
h) 0.59 mg/l (nominal concentration). Algae Does not significantly
affect the average specific growth rate of Selenastrum capricornutum.
Bees LD50 (oral) 0.55 mg/bee. Worms LC50 (14 d) 38 mg/kg soil.
Pyridaben ENVIRONMENTAL FATE
Animals In the rat, goat and hen, an orally administered dose is
excreted mainly in the faeces. Metabolism is complex, with at least
30 degradates. Plants After application to citrus and apple, pyridaben
degrades gradually photochemically and is not translocated into
the pulp. Soil/Environment Readily degrades microbiologically in
aerobic soils, DT50 <21 d; further degrades to polar products
(including soil-bound residues) and CO2. In natural water, DT50
10 d (25 C, in dark). DT50 for aqueous photolysis c. 30 min (pH
7). See also Stability.